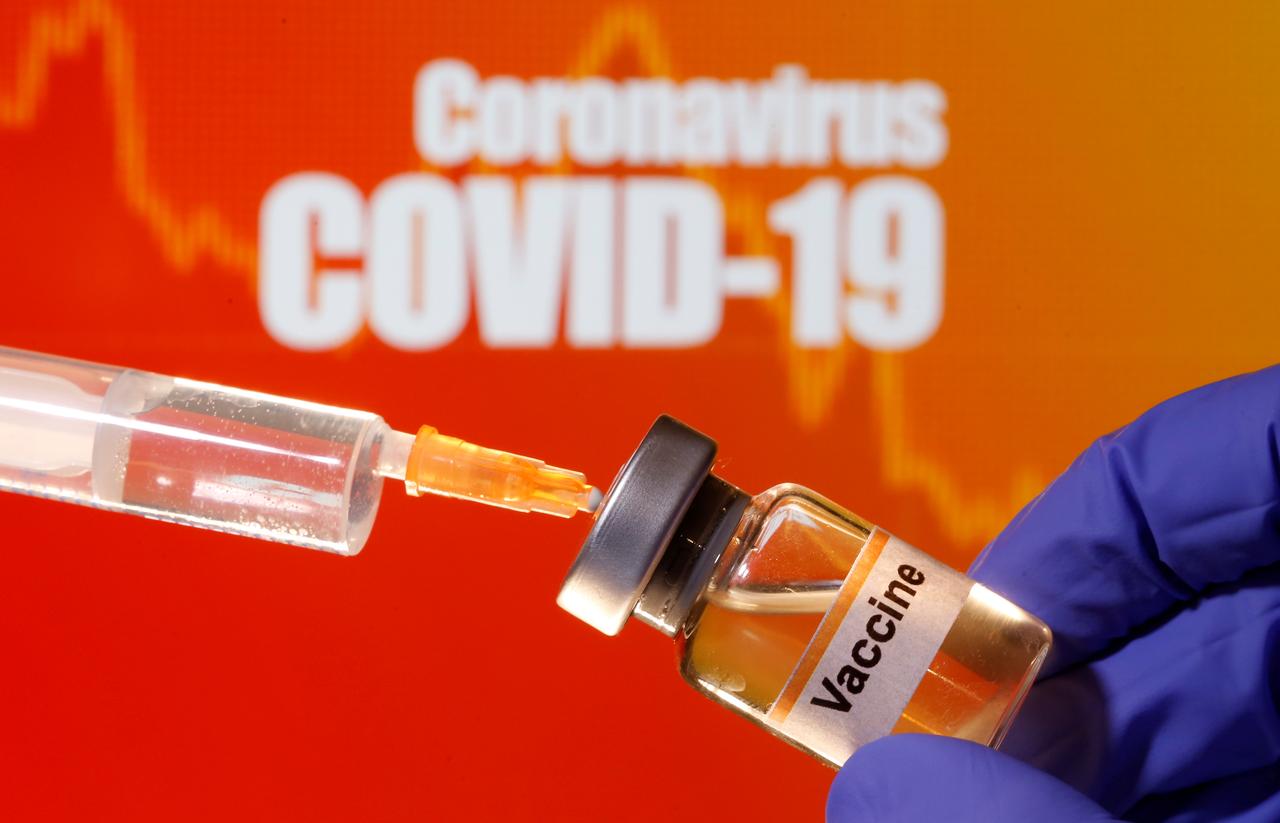
Image credit: Dado Ruvic/Reuters
Lexa Krug, contributing
New York City & Mainz, Germany – The U.S. government has struck a $1.95 billion deal with American pharmaceutical company Pfizer and German biotechnology company BioNTech for 100 million COVID-19 vaccine doses. This deal can extend to an additional 500 million doses. If trials are successful, the vaccines can be ready for Emergency Use Authorization as early as October 2020. The transaction continues America’s Operation Warpspeed, the U.S. funded project that aims to have 300 million vaccine doses by January 2021.
The development of possible vaccines continues through various means. Biotechnology firm Moderna, in coordination with the National Institutes of Health (NIH), and Pfizer both entered phase three trials on Monday, July 27. Novavax, a vaccine development company, finished phase 1 of testing and will likely begin phase 2 this month, Novavax spokesperson Edna Kaplan told the Current Affairs Times. Biopharmaceutical corporation AstraZeneca will begin phase 3 trials in a few weeks. Johnson & Johnson subsidiary Jansen has developed a successful vaccine for primates.
Like what you’re reading? Consider subscribing to access our premium content!
Operation Warp Speed is searching for vaccines with evidence of both clinical potential and early timing of a vaccine, so these vaccines can be distributed in late 2020 or early 2021. In a July 30 Operation Warp Speed briefing, an anonymous senior administration official reported that Operation Warp Speed only pays for vaccines from pharmaceutical companies if the Emergency Use Authorization is activated or licensure is obtained from the FDA. This means that companies “put themselves at risk, not the US government and its taxpayer[s] and we feel very good about that contract.”
With the development of various coronavirus vaccines from around the world, will the supply be able to meet the intense demand from the public? Will Operation Warp Speed be able to distribute a vaccine by the end of 2020? And will these early vaccines be effective enough to combat the pandemic?
Image credit: Dado Ruvic/Reuters
Sources:
- Pfizer, BioNTech reach $1.95 billion covid-19 vaccine deal with U.S. government
- Pfizer and BioNTech Announce an Agreement with U.S. Government for up to 600 Million Doses of mRNA-based Vaccine Candidate Against SARS-CoV-2
- Edna Kaplan, Novavax spokesperson
The July 30 Operation Warp Speed briefing was provided to the Current Affairs Times by a spokesperson from the Department of Health and Human Services.